The nitrogen cycle is a complex biogeochemical process that transforms inert atmospheric nitrogen into various compounds, making it available to living organisms. This cycle is integral for life on Earth and is influenced by both natural processes and human activities.
Introduction
Nitrogen is essential for the synthesis of amino acids, proteins, and nucleic acids, which are the building blocks of life. However, the majority of organisms cannot directly utilise atmospheric nitrogen. The nitrogen cycle encompasses the series of transformations that nitrogen undergoes to become bioavailable, and how it is eventually returned to the atmosphere.
Key Processes of the Nitrogen Cycle
Nitrogen Fixation
Nitrogen fixation is the conversion of atmospheric nitrogen (N2) into ammonia (NH3), making it accessible to plants.
Biological Fixation: Specific bacteria, such as Rhizobium, and cyanobacteria have the unique ability to fix atmospheric nitrogen. They often live symbiotically with leguminous plants, providing them with nitrogen in exchange for carbohydrates.

Close-up of nitrogen-fixing nodules on legume roots formed by Rhizobium bacteria. This real-world image illustrates biological nitrogen fixation referenced in the notes; it does not depict other cycle steps. Source.
Atmospheric Fixation: Lightning facilitates the reaction between nitrogen and oxygen, forming nitrogen oxides (NOx) that dissolve in rain, leading to the formation of nitrates.
Industrial Fixation: The Haber-Bosch process is a significant contributor to nitrogen fixation, synthesising ammonia from nitrogen and hydrogen gases under high pressure and temperature, primarily for agricultural fertilisers.
Nitrification
This two-step process involves the conversion of ammonia to nitrite and subsequently to nitrate.
Ammonia to Nitrite: Specialised bacteria like Nitrosomonas oxidise ammonia to form nitrite. This process is essential as ammonia can be toxic to plants in high concentrations.
Nitrite to Nitrate: Nitrobacter bacteria facilitate the conversion of nitrite to nitrate, which plants can readily absorb.
Assimilation
Plants take up ammonia and nitrate from the soil and convert these into amino acids, proteins, and other essential biological molecules.
Animals, in turn, obtain these nitrogenous compounds by consuming plants or other animals.
Ammonification
Decomposers, including certain bacteria and fungi, break down dead organisms and waste materials, converting the contained organic nitrogen into ammonia, which re-enters the soil.
Denitrification
Denitrifying bacteria, such as Pseudomonas, convert nitrates and nitrites in the soil back into nitrogen gas or nitrous oxide, releasing them into the atmosphere and completing the cycle.
Human Influences on the Nitrogen Cycle
Agricultural Practices
Fertiliser Use: The application of nitrogen-rich fertilisers enhances plant growth but also introduces an excess of reactive nitrogen into ecosystems, leading to imbalances and pollution.
Irrigation: Excessive irrigation can lead to the leaching of nitrates into groundwater and surface water bodies.
Industrial Activities
Combustion of Fossil Fuels: This releases a significant amount of nitrogen oxides into the atmosphere, contributing to air pollution, acid rain, and climate change.
Haber-Bosch Process: While beneficial for food production, this process has led to a significant increase in reactive nitrogen in the biosphere.
Waste Management
Sewage and Waste Disposal: Inadequate treatment and disposal practices can lead to the release of large amounts of nitrogen compounds into rivers and oceans.
Ecological Impacts of Human Activities
Eutrophication
The influx of nitrogen, particularly from agricultural runoff, into aquatic ecosystems can lead to eutrophication, where an excess of nutrients causes rapid algal and plant growth.
The subsequent decomposition of these organisms by bacteria depletes oxygen levels in the water, leading to hypoxic conditions and the death of aquatic life.
Soil Acidification
The overuse of nitrogenous fertilisers can lead to the acidification of soils, negatively impacting plant growth, soil structure, and microbial activity.
Air Pollution
Nitrogen oxides are a primary component of smog and contribute to respiratory problems in humans and other animals.
Climate Change
Nitrous oxide is a potent greenhouse gas with a significant global warming potential, contributing to the enhanced greenhouse effect and climate change.
Biodiversity Loss
Altered nitrogen levels in ecosystems can lead to shifts in species composition, favouring nitrogen-loving species and leading to a reduction in biodiversity.
Mitigating Human Impacts
Sustainable Agricultural Practices
Reduced Fertiliser Use: Utilising precision agriculture techniques to apply fertilisers more efficiently, minimising waste and environmental impact.
Crop Rotation: Incorporating leguminous plants that host nitrogen-fixing bacteria can naturally enrich the soil.
Pollution Control
Technological Innovations: Advancements in technology can reduce nitrogen oxide emissions from vehicles and industrial processes.
Waste Treatment: Improving waste treatment facilities to effectively remove nitrogen compounds from wastewater.
Policy and Regulation
Legislation: Implementing and enforcing strict regulations on emissions and chemical use to mitigate the impact on the nitrogen cycle.
International Cooperation: Collaborating on a global scale to address the transboundary impacts of nitrogen pollution.
Case Study: The Gulf of Mexico Dead Zone
Each year, the Gulf of Mexico experiences a significant “dead zone” where oxygen levels plummet, primarily due to excessive nutrient pollution, including nitrogen, from the Mississippi River.
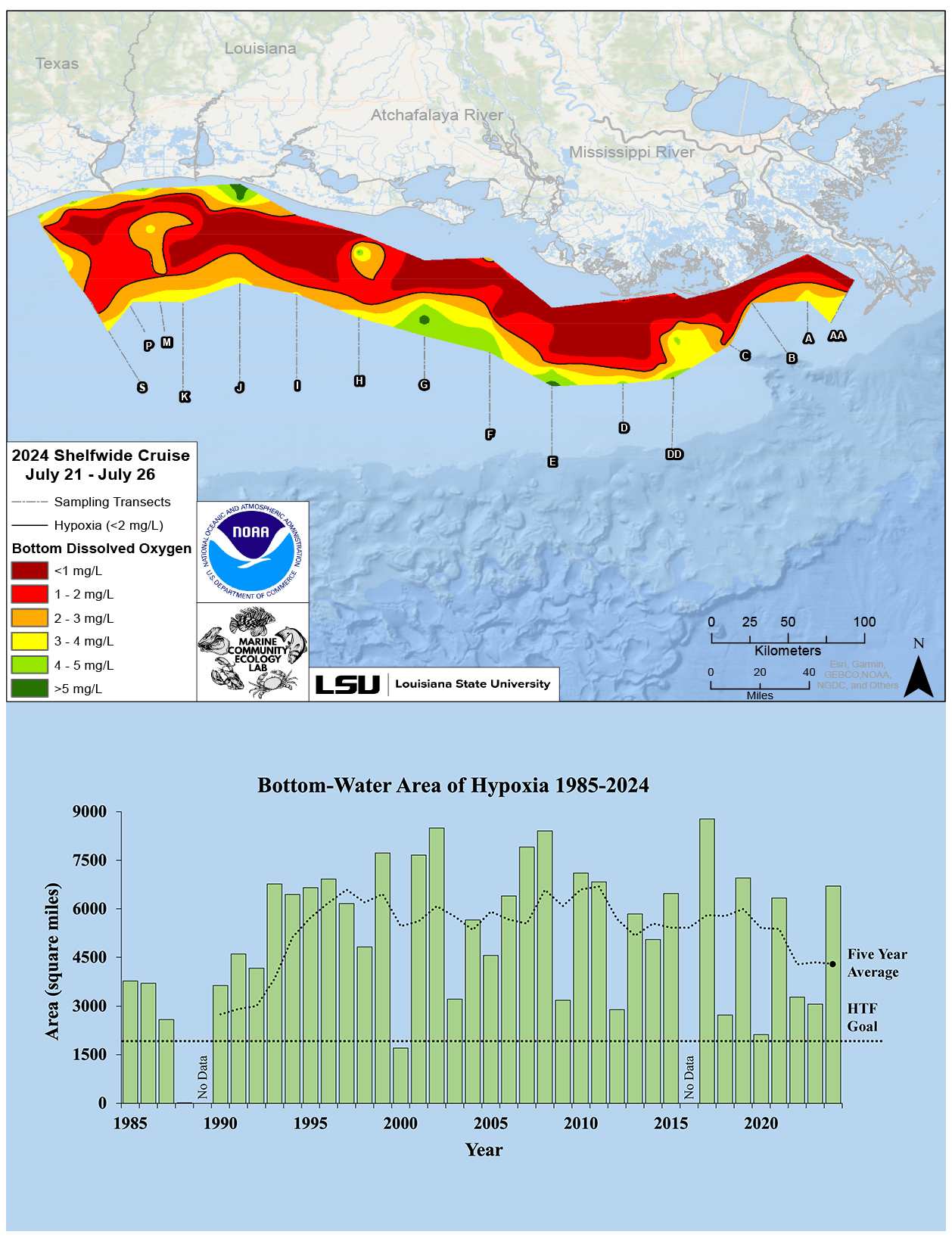
Map of the 2024 hypoxic zone along the northern Gulf coast with a legend for bottom dissolved oxygen; below it, a bar chart shows hypoxic area by year since 1985. The long-term bar chart provides extra context beyond the syllabus but clarifies the scale and variability of the case study. Source.
Causes
Agricultural Runoff: The intensive agriculture in the Midwest contributes a significant load of nitrogen to the Mississippi, leading to nutrient pollution in the Gulf.
Urban Runoff: Cities contribute to the problem through stormwater and wastewater discharge.
Impacts
Marine Life: The hypoxic conditions lead to a significant loss of biodiversity, with many species dying or migrating to more hospitable areas.
Fisheries: The fishing industry faces severe impacts due to the loss of fish and other marine resources.
Solutions
Riparian Buffers: The establishment of vegetated areas along rivers can help absorb and filter out excess nutrients.
Wetland Restoration: Wetlands act as natural filters for nutrients, and their restoration and preservation are crucial for mitigating nutrient pollution.
In the intricate dance of the nitrogen cycle, every step, from fixation to denitrification, plays a critical role in sustaining life on Earth. The human influence, while monumental, poses significant challenges and threats to the delicate balance of this cycle. Understanding these processes and impacts is the first step towards developing solutions for a sustainable future.
FAQ
The nitrogen and carbon cycles are intricately linked in ecosystems. Nitrogen is essential for plant growth and is a key component of proteins and nucleic acids. As plants absorb carbon dioxide for photosynthesis, they also require nitrogen to synthesise organic molecules. The availability of nitrogen can therefore limit primary productivity and influence carbon sequestration in ecosystems. In aquatic systems, an excess of nitrogen can lead to eutrophication, where increased algal growth and subsequent decomposition lead to reduced oxygen levels and the release of carbon dioxide. Thus, alterations to the nitrogen cycle can have cascading effects on the carbon cycle and overall ecosystem functioning.
The Haber-Bosch process, which synthesises ammonia from nitrogen and hydrogen gases, has significantly increased the amount of reactive nitrogen available in the biosphere. While this has boosted agricultural productivity, it has also led to environmental issues. The excessive use of nitrogen-based fertilisers has resulted in nutrient runoff into water bodies, causing eutrophication and dead zones. Additionally, the release of nitrogen oxides during the industrial production and combustion processes contributes to air pollution, acid rain, and climate change. The increased availability of reactive nitrogen has also led to soil acidification and a loss of biodiversity, as ecosystems struggle to adapt to the altered nutrient levels.
Individuals and communities can reduce their impact on the nitrogen cycle through various means. Practising sustainable agriculture, such as using fertilisers efficiently and adopting crop rotation with nitrogen-fixing plants, can minimise nitrogen runoff. Reducing the consumption of energy, especially from fossil fuels, can lower nitrogen oxide emissions. Proper waste management, including composting and recycling, can decrease the amount of nitrogen waste entering landfills and water bodies. Additionally, supporting policies and initiatives that aim to regulate and reduce nitrogen emissions, promote conservation, and enhance the sustainable management of nitrogen can contribute to mitigating the human impact on the nitrogen cycle.
Nitrogen-fixing plants, particularly legumes, play a vital role in the nitrogen cycle by hosting symbiotic bacteria in their root nodules that can convert atmospheric nitrogen into ammonia, a form that plants can utilise. This process, known as biological nitrogen fixation, enhances soil fertility by increasing the availability of nitrogen compounds. These plants act as a natural fertiliser, promoting plant growth and productivity in nitrogen-poor soils. The increased nitrogen availability also supports biodiversity, as various plant and animal species benefit from the enriched nutrient content in the ecosystem, leading to more complex and resilient communities.
The nitrogen cycle contributes to acid rain primarily through the release of nitrogen oxides (NOx) into the atmosphere. These gases are produced from various sources, including the combustion of fossil fuels in vehicles, power plants, and industrial processes. When released into the atmosphere, nitrogen oxides react with water vapour and other chemicals to form nitric acid. This acid is then carried by atmospheric currents and can fall to the ground as acid rain when it combines with precipitation. Acid rain has detrimental effects on ecosystems, including soil acidification, damage to aquatic life, and the degradation of vegetation and built structures.
Practice Questions
Agricultural practices, particularly the excessive use of nitrogen-based fertilisers, significantly impact the nitrogen cycle. They introduce an abundance of reactive nitrogen into ecosystems, leading to imbalances and pollution. This excess nitrogen can cause eutrophication in aquatic ecosystems, where an overabundance of nutrients leads to rapid algal growth. The subsequent decomposition of these algae by bacteria depletes oxygen levels in the water, leading to hypoxic conditions and the death of aquatic life. Soil health is also affected, with an overabundance of nitrogen leading to soil acidification, impacting plant growth and soil microorganisms.
A sustainable solution to mitigate these impacts is the implementation of precision agriculture. This practice involves the precise application of fertilisers, ensuring that crops receive the exact amount of nutrients they need, minimising waste and environmental pollution. It utilises advanced technologies to monitor and manage field variability in crops, enabling the efficient use of resources. By reducing the overapplication of fertilisers, precision agriculture can significantly decrease the runoff of nitrogen into nearby water bodies, mitigating eutrophication and soil acidification, and promoting overall ecosystem health.
Denitrifying bacteria play a crucial role in the nitrogen cycle by converting nitrates and nitrites in the soil back into nitrogen gas or nitrous oxide, releasing them into the atmosphere. This process, known as denitrification, is essential for maintaining the balance of nitrogen in ecosystems. It prevents the accumulation of excess nitrates in the soil, which can lead to issues like eutrophication and groundwater contamination. Denitrifying bacteria, including species like Pseudomonas and Clostridium, facilitate this conversion under anaerobic conditions, often found in waterlogged soils and aquatic sediments.
However, the activity of denitrifying bacteria also has implications for the environment. While they help in reducing the concentration of nitrates in the soil and water, they release nitrous oxide (N2O) into the atmosphere, a potent greenhouse gas with a significant global warming potential. Nitrous oxide contributes to the enhanced greenhouse effect and climate change, trapping heat in the Earth’s atmosphere. It is also involved in the depletion of the ozone layer. Balancing the beneficial aspects of denitrification with the environmental impacts of nitrous oxide emissions is a complex challenge in managing the nitrogen cycle sustainably.

