The laws of thermodynamics are integral to understanding the intricate dynamics of energy within environmental systems. These foundational principles offer insights into energy conservation, transformation, and entropy, playing a pivotal role in shaping the ecosystems.
First Law of Thermodynamics - Energy Conservation
The first law, often referred to as the law of energy conservation, posits that energy in a closed system remains constant. It can neither be created nor destroyed but can only change forms. This principle is paramount in studying energy flow and transformations within ecosystems.
Energy Transformation
Types of Energy: Energy manifests in various forms like kinetic, potential, thermal, and chemical. In environmental systems, energy transformations are commonplace, with solar energy being converted into chemical energy by plants, which is then transformed into other forms as it moves along the food chain.
Energy Flow: Energy’s journey from the sun to producers and then to consumers and decomposers is a complex process involving multiple transformations. Each transformation adheres to the first law, ensuring energy conservation. For a deeper understanding of how energy transfers within ecosystems, explore our detailed notes on energy transfers.
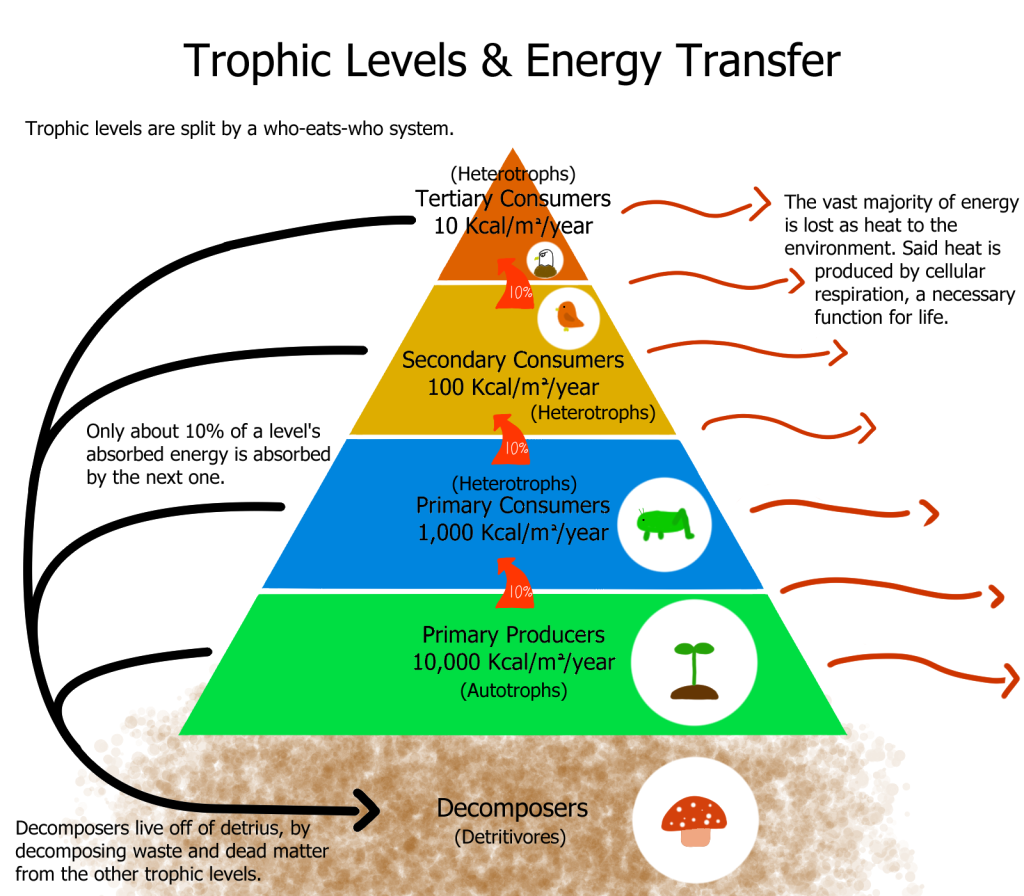
This diagram shows energy moving through trophic levels, with decreasing usable energy and heat loss at each transfer. It reinforces conservation of energy while illustrating the entropy increase that constrains ecological efficiency. Source.
Energy Balance
Input and Output: Every environmental system has a distinct energy input, output, and internal energy change. The first law ensures that energy is accounted for in these processes, maintaining a balance that is crucial for the system’s stability.
Ecosystem Productivity: The energy available in an ecosystem determines its productivity. The first law aids in quantifying this energy, offering insights into primary and secondary productivity levels. Discover more about these productivity measures in our notes on primary and secondary productivity.
Second Law of Thermodynamics - Energy Quality
The second law introduces the concept of energy quality, emphasizing that energy transformations are not perfectly efficient. It introduces entropy, a measure indicating the level of energy dispersion in a system.
Entropy
Energy Dispersion: Energy becomes more dispersed and less available for work as it transforms. This increased dispersion, or entropy, is a natural tendency in all energy transformations. The dispersion of energy also influences the nutrient cycles in an ecosystem, affecting nutrient availability and ecosystem health.
Natural Processes: Every natural process, from the growth of plants to the movement of planets, increases the universe’s total entropy. This law underscores the inherent inefficiency in energy transformations.
Implications for Environmental Systems
Energy Efficiency: Energy transfers within ecosystems are not 100% efficient. A significant portion is always lost as heat, a direct application of the second law, leading to increased entropy in the surroundings.
Trophic Levels: The energy losses at each trophic level, resulting in the typical pyramid structure of ecosystems, can be explained by the second law.
Ecosystem Dynamics
Nutrient Cycling: The flow of energy through nutrient cycles is influenced by the second law. It affects the availability and form of nutrients, impacting ecosystem health and productivity.
Ecological Succession: The dynamics of ecological succession, including the energy transformations and entropy increases, are governed by the second law.
Relevance to Environmental Systems
The first and second laws of thermodynamics are instrumental in dissecting the energy dynamics within environmental systems. These laws influence everything from the microscopic interactions within cells to the global patterns of climate and weather.
Ecosystem Energy Flow
Energy Transfer: The first law illuminates the conservation aspect during energy transfers between trophic levels. In contrast, the second law explains the energy losses and increased entropy associated with these transfers.
System Stability: The energy balance, a direct implication of the thermodynamic laws, is integral to the stability, resilience, and functioning of ecosystems.
Environmental Changes
Climate Change: These laws are pivotal in comprehending the energy imbalances leading to global climate change. They help in quantifying the energy trapped in the atmosphere, leading to increased temperatures. The impact of these energy dynamics is also pivotal in the development of solar energy technologies.
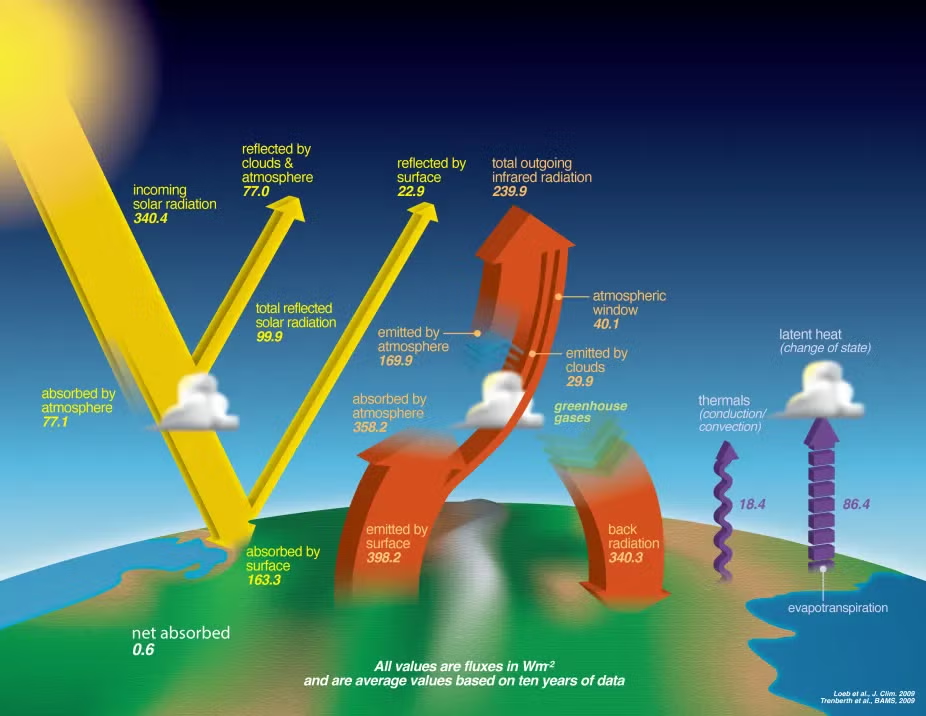
This diagram summarises Earth’s radiation budget, contrasting incoming solar radiation with reflected and emitted energy and the greenhouse effect. Values provide a quantitative view of energy imbalance, illustrating how small departures from balance drive warming (more detail than required by the syllabus; focus on the balance concept). Source.
Resource Management: Efficient management of natural resources is anchored in the understanding of energy transformations and losses, as explained by the thermodynamic laws.
Biodiversity
Species Interactions: The energy interactions among species, influenced by these laws, affect the patterns of biodiversity within ecosystems.
Ecosystem Services: The flow and transformation of energy within ecosystems, governed by the thermodynamic laws, underpin the provision of essential ecosystem services.
Practical Applications
The laws of thermodynamics aren’t just theoretical constructs but have practical applications in environmental management and conservation. They are the bedrock upon which strategies for energy efficiency and sustainability are built.
Conservation Strategies
Energy Efficiency: Strategies to augment energy efficiency in various sectors are rooted in the principles of thermodynamics. They guide the optimization of energy use, minimizing losses and enhancing sustainability.
Renewable Energy: The development of renewable energy sources is informed by the laws of thermodynamics. They aid in assessing the energy output and efficiency of various renewable energy technologies.
Environmental Policies
Sustainability: Policies aiming for sustainable development are grounded in the thermodynamic principles, ensuring that energy and resource use aligns with the Earth’s carrying capacity.
Climate Action: The laws of thermodynamics are foundational in formulating strategies to mitigate and adapt to climate change, addressing the energy imbalances in the atmosphere.
In the realm of environmental systems, the first and second laws of thermodynamics are not abstract, isolated concepts. They are intricately woven into the fabric of ecosystems, influencing their structure, function, and responses to environmental changes. Every energy transfer, transformation, and interaction within the environment echoes the principles encapsulated in these laws. Understanding them is not just an academic exercise but a prerequisite for informed, effective action towards a sustainable future.
FAQ
The second law of thermodynamics, stating that systems tend towards increased entropy, cannot be reversed or violated in any isolated system. In both natural and artificial processes, energy tends to disperse and become less ordered over time. For instance, in energy transfers within an ecosystem or in a machine, some energy is always lost as heat, contributing to increased entropy. While humans can create local decreases in entropy (e.g., the ordering of energy in a battery), these are always at the expense of increased entropy elsewhere, ensuring the universal applicability of the second law.
The second law of thermodynamics elucidates the inherent inefficiency in energy transfers within ecosystems. As energy moves through trophic levels—from producers to primary consumers, and then to higher-order consumers—a significant portion is lost as heat. This is a direct manifestation of the second law, which posits an increase in entropy or disorder during energy transformations. Consequently, only a fraction of the energy is passed on to the next trophic level, leading to the observed decrease in available energy along the food chain and contributing to the pyramid structure of ecosystems.
The laws of thermodynamics are fundamental in shaping human energy use and technologies. The first law, emphasizing energy conservation, underscores the need for efficient energy use and the development of technologies that minimize energy loss. The second law, highlighting the tendency for increased entropy, explains the inherent losses and inefficiencies in all energy transformations, from electricity generation to automobile engines. These laws guide the engineering and innovation of more efficient and sustainable energy technologies, driving advancements in renewable energy, energy storage, and energy conservation practices to mitigate environmental impacts.
The first law of thermodynamics is akin to the law of conservation of matter, as both principles assert a constancy in the total quantity of their respective subjects—energy and matter. In environmental systems, this means that while energy and matter undergo various transformations and transfers, their total amount within a closed system remains unchanged. For instance, in a pond ecosystem, the matter that makes up plants, animals, and microorganisms is constantly cycling, yet the total mass remains constant. Similarly, the energy from the sun is converted into chemical energy and then transferred through the food web, but the total energy is conserved.
The laws of thermodynamics directly impact the structure of food chains and webs. The first law ensures that energy is conserved at each trophic level, but the second law introduces the concept of energy loss due to increased entropy. As a result, less energy is available at each successive trophic level, leading to shorter food chains and a pyramid structure of biomass and energy in ecosystems. This energy loss also influences the complexity of food webs, as it limits the number of trophic levels and the potential for diverse interactions among species within an ecosystem.
Practice Questions
The first law of thermodynamics, or the law of energy conservation, is exemplified in ecosystems through the constant transformation of energy from one form to another without any loss in total energy. For instance, in a forest ecosystem, solar energy is transformed into chemical energy by plants during photosynthesis. This stored chemical energy is then transferred to herbivores when they consume plants, and further to carnivores as they prey on herbivores. Throughout these energy transfers and transformations, the total energy within the ecosystem remains constant, underscoring the first law of thermodynamics.
The second law of thermodynamics, which posits that energy disperses and becomes less available for work as it transforms, impacts energy transfers between trophic levels by introducing inefficiency. In an ecosystem, as energy moves from producers to consumers and then to decomposers, a significant portion is lost as heat at each trophic level. This phenomenon explains the diminished energy available at successive trophic levels, leading to the pyramid structure of ecosystems. The energy lost as heat increases the overall entropy of the system, aligning with the second law’s assertion of a natural tendency towards increased disorder.

